A mutation that can cause autism
Read a summary of how researchers from UiB investigate new genetic variants associated with intellectual disability, autism spectrum disorder, and congenital abnomalies.

Main content
Dr. Sylvia Varland and Professor Thomas Arnesen from the Department of Biomedicine at the University of Bergen have helped investigate new genetic variants associated with intellectual disability, autism spectrum disorder, and congenital anomalies, as part of an international team of physicians and researchers led by Dr. Gholson Lyon at the Cold Spring Harbor Laboratory.
The study reported in the American Journal of Human Genetics identified and phenotypically characterized 37 individuals from 32 unrelated families with 25 different and likely gene disruption variants in NAA15 (OMIM: 608000), which is emerging as a genetic risk factor for Autism Spectrum Disorder (ASD).
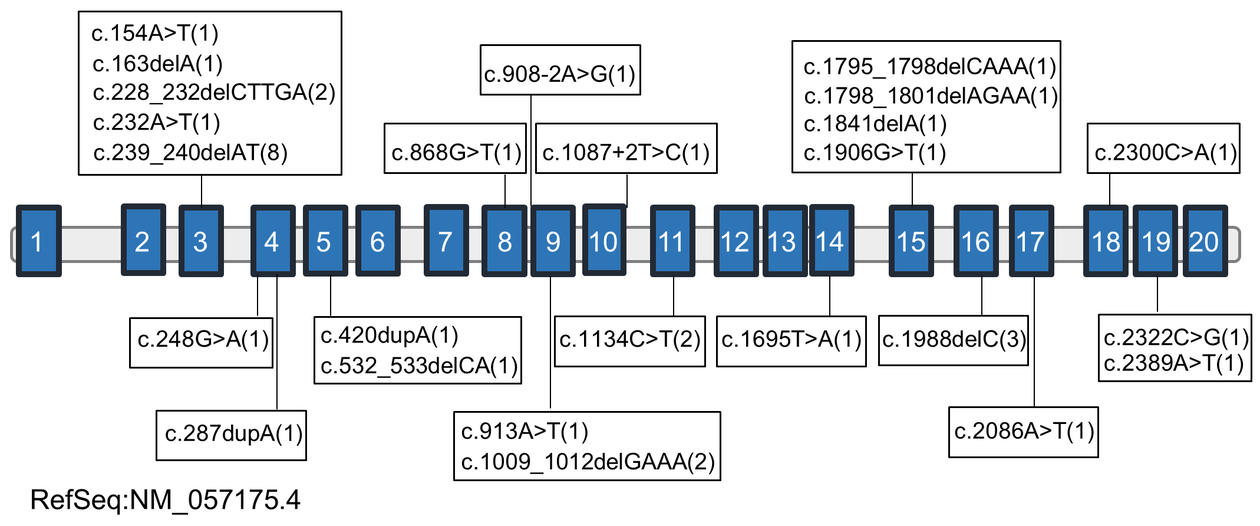
All the affected individuals have variable degrees of neurodevelopmental disabilities, including intellectual disability, delayed speech and motor milestones, and ASD. The various genetic variants of NAA15 are contained in twelve of the twenty exons and two intron/exon boundaries (see figure in the side bar). The authors determined that the genetic mutations in most cases appeared spontaneously in the individual’s genome (known as de novo mutations), whereas familial inheritance was observed in three families. Among the 25 variants identified there were three recurrent variants (p.Asp76Glufs, p.His80Argfs, and Glu337Argfs), which occurred in so-called mutation hotspots.
To reduce errors in gene expression our cells have developed surveillance systems that recognize and eliminate mRNA transcripts that contain premature stop codons. The process is known as nonsense-mediated mRNA decay (NMD). RNA analysis from two of the patients showed degradation of the mutated transcripts, likely due to NMD.
NAA15 is part of an enzyme complex called NatA, which modifies the very beginning of many proteins in our bodies. The widespread protein modification is known as N-terminal acetylation and is the research focus of the research group led by Professor Thomas Arnesen.
“These new NAA15 mutations expand the number of gene variants that are associated with intellectual disability and autism spectrum disorder and they represent new avenues of research to explore how N-terminal acetylation affects human health and disease”, says Thomas Arnesen.
In order to shed light on the functionality of the novel NAA15 mutations, Sylvia Varland used baker’s yeast as a model organism. The human NatA complex can functionally replace yeast NatA, as shown by complementation of growth phenotypes and partial rescue of NatA-specific Nt-acetylome. She was able to show that two mutant variants of NAA15 (p.Thr55Hisfs and p.Lys305*) failed to rescue the temperature sensitive growth phenotype in yeast lacking NatA, suggesting that the two variants lead to reduced or abolished NatA activity. With the RNA analysis in mind is not unreasonable to believe that the truncated mini-versions of NAA15 are subject to NMD. Additional functional work is required to further elucidate these genotype-to-phenotype relationships.
“Yeast is a powerful model organism to study human disease”, says Sylvia. “These little creatures that help us raise bread and brew beer are not as genetically different to us humans as one might think. Considering the ease of genetic manipulation, short generation time, and a helpful yeast community, yeast is my model organism of choice”, she continues.
Genetic defects in NAA10 (OMIM: 300013), which is X-linked and encodes the catalytic subunits of the NatA complex, are associated with the infantile lethal Ogden syndrome, Lenz micropthalmia, intellectual disability, and cardiac abnormalities. Missense mutations in NAA10 are reported to decrease the enzymatic activity of NAA10 and/or alter complex formation with NAA15. Individuals carrying mutations in NAA10 and NAA15 appear to have some phenotypic overlap, but also show extensive phenotypic variability. The authors therefore suggest that functional deficiency of the two gene products should be referred “NAA10-related syndrome” and “NAA15-related syndrome”.
About the authors
Dr. Sylvia Varland obtained a Ph.D. in Molecular Biology at the University of Bergen in 2016. She received a personal mobility grant fellowship from the Research Council of Norway and is currently working as a PostDoc in the Boone and Andrews lab at the Donnelly Centre for Cellular and Biomolecular Research, University of Toronto, Canada.
Professor Thomas Arnesen is affiliated with the Department of Biomedicine and Department of Biological Sciences at University of Bergen, Department of Surgery at Haukeland University Hospital, and is a young Associate Investigator at the Norwegian Center for Molecular Medicine. He recently received the ERC Consolidator Grant and is also funded by the Research Council of Norway, the Norwegian Cancer Society, and the Norwegian Health Authorities of Western Norway.