Christiaen Group
We use CRISPR/Cas9, imaging, single cell genomics and modeling to study multipotent cardiopharyngeal progenitors and cellular behaviors in the ascidian Ciona.

Hovedinnhold
During animal development, tissues and organs assume shapes and positions that are largely determined by the species-specific genetic blueprint. These macroscopic events are powered by a variety of cells that divide, change shape and move in response to cell-cell communications and lineage-specific genetic programs. Our overarching goal is to reach a system’s level understanding of how tissue-specific regulatory programs and cell-cell communications coordinate the fate choices and cellular behaviors that underlie animal development, regeneration and evolution.
We focus on cardiopharyngeal lineages, which produce both cardiomyocytes of the second heart field and head muscles from Mesp1+ anterior mesoderm progenitors. The cardiopharyngeal paradigm casts new light on certain congenital diseases characterized by the co-occurence of cardiac and craniofacial defects, such as the DiGeorge/22q11.2 deletion syndrome. The latter is thought to emerge from haploinsufficiency of TBX1, a key determinant of cardiopharyngeal progenitors. This illustrates the biomedical relevance of our work on the regulation of cardiopharyngeal multipotency and early heart vs. pharyngeal muscle fate choices.
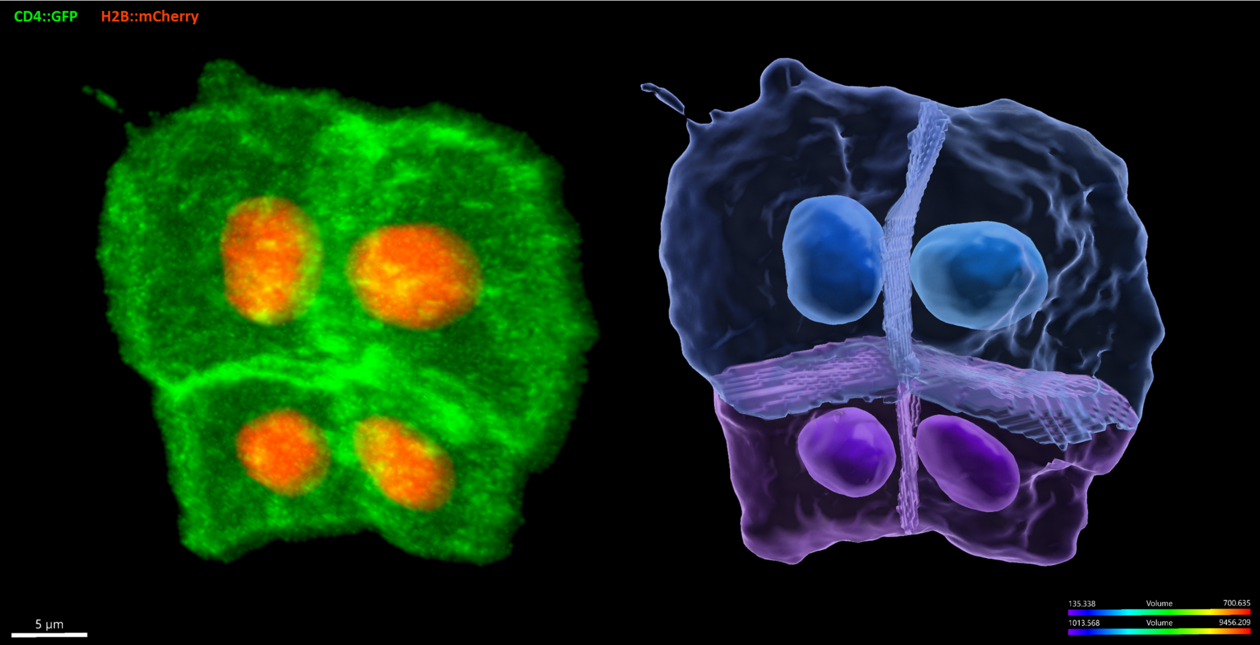
Circumventing the complexity of vertebrates, we use embryos and larvae of the tunicate Ciona to study cardiopharyngeal development with high spatial and temporal resolution. In ascidians like Ciona, every invariant division and migratory event is mapped onto a stereotyped and evolutionarily conserved developmental sequence. We strive to develop state-of-the-art methods to study Ciona development, including targeted genome engineering with CRISPR/Cas9, single cell genomics, quantitative imaging and mathematical modeling. Leveraging the simplicity of the Ciona model and our extensive experimental toolkit, we pursue a system’s level understanding of cellular behaviors with a focus on collective polarity, directed migration, and oriented and asymmetric cell divisions.
Finally, we are expanding the scope of our research towards (1) the evolution of cardiopharyngeal development through comparative studies in tunicates and vertebrates, including mammalian stem cell models; (2) the regenerative potential of cardiopharyngeal structures in tunicates and their relationships with other endomesodermal systems; and (3) environmentally-relevant problems in developmental systems biology.