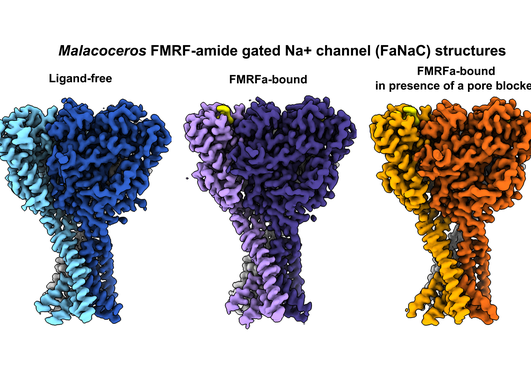
Cross-phyla insights into iGluR function: From rat NMDA receptors to placozoan leak channels
Researchers from the Lynagh Group reveal how minor amino acid variations in ionotropic glutamate receptors (iGluRs) can lead to major functional changes across animal phyla.

Main content
In a recent publication in the journal Structure, a team of researchers including Sandra Seljeset, Oksana Sintsova, Yuhong Wang, and Timothy Lynagh from the Lynagh Group has provided new insights into the function of neurotransmitter receptors, particularly ionotropic glutamate receptors (iGluRs). Their detailed studies on rat NMDA receptors and the discovery of analogous, naturally occurring mutations in placozoan iGluRs highlight the adaptability of these crucial neural components and open up new pathways for both biomedical and biological research.
Detailed Exploration of NMDA Receptors
At the heart of this research is the detailed exploration of NMDA receptors, a critical component of the neural signaling mechanisms in mammals, including humans. Researcher Oksana Sintsova, building on previous work by former postdoc Sandra Seljeset, employed site-directed mutagenesis and biophysical measurements of heterologously expressed receptors, to induce curious changes in NMDA receptor behavior.
"I continued the detailed investigation of rat NMDA receptors, including site-directed mutagenesis, expressing receptors heterologously in frog eggs in the lab, and then biophysical/pharmacological measurement of receptor function," explained Oksana. The most striking aspect of her findings was that a single amino acid substitution could transform the receptor into a constantly active state.
This paper sets a precedent for successfully predicting natural receptors with previously unobserved functional features based on insights we gained from studying multiple laboratory-generated mutations.
- Oksana Sintsova
Discovery of a Natural Analog in Placozoans
Parallel to Oksana’s research, Yuhong investigated the iGluR gene family across different species, eventually focusing on Trichoplax adhaerens, a simple placozoan with no conventional nervous system. Surprisingly, she identified a naturally occurring mutation in these placozoan iGluRs similar to the one Oksana studied in rat iGluRs. When expressed and tested, these receptors also exhibited constitutive activity, confirming the predictive power of the hypothesis emerging in the first part of the work.
"In this placozoan receptor, there is a natural substitution at an otherwise highly conserved carboxylate amino acid residue in the ligand-binding domain, which helps us understand the role of this residue in channel activity," Yuhong stated. This finding was unexpected in the context of typical neurotransmitter-dependent iGluR activity, and groundbreaking in revealing a clear link in the molecular determinants of neuronal function between placozoans and vertebrates.
Implications and Future Directions
These discoveries offer a new understanding of how iGluRs can evolve to acquire new functions, such as becoming leak channels that are active without neurotransmitter triggers.
“On a biophysical level, we learn something about how neurotransmitter receptors work, and on a biological level we learn that certain animals have leak channels that evolved out of neurotransmitter receptors via particular mutations in the neurotransmitter-binding site,” said Tim. This knowledge could pave the way for developing animal models exploring the roles of different neurotransmitters in neural signaling.
Both Yuhong and Oksana underscored the importance of advanced methodologies in their work. Oksana highlighted the role of genetic databases in forming hypotheses about protein functions, while Yuhong noted the use of electrophysiology and mutagenesis in elucidating the interaction dynamics within iGluRs.
Reflecting on the publication process, Oksana appreciated the innovative peer review service that expedited their publication: "We used a new service for an independent open review of our paper provided by Biophysics Colab. After improvements were made based on that open review, our paper was accepted by the journal Structure without additional review. We were very pleased with this experience."
This study not only enhances our understanding of neurotransmitter receptor function but also demonstrates the unexpected ways in which the study of simple marine organisms can inform us on complex biological mechanisms in higher animals.