Actin Modifications: Finding the Right Shoe for Cinderella
Cinderella is a tale of being lifted from obscurity to recognition and significance. A review by young researchers at the Department of Biomedicine highlights the importance of the post-translational modifications of actin.

Main content
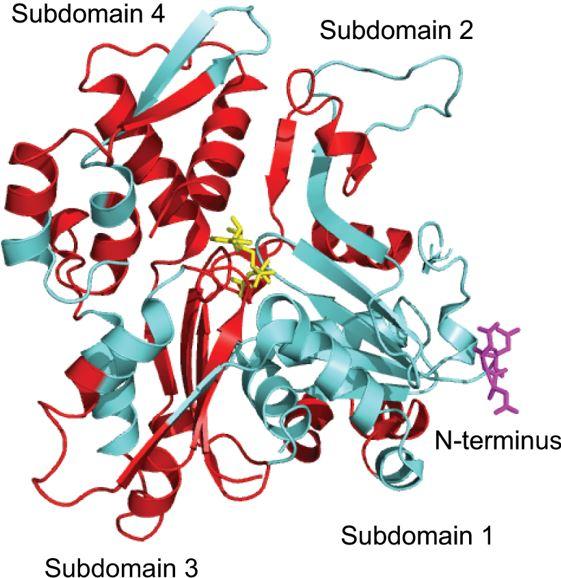
Actin is one of the most abundant proteins in our bodies and the main component of the cytoskeleton. It is involved in numerous cellular activities such as muscle contraction and cell motility. Sylvia Varland and Adrian Drazic are young researchers at the Department of Biomedicine and have joined forces with actin expert Joël Vandekerckhove from the University of Ghent in Belgium. In their new review article they describe the post-translational modifications of actin.
"Such a versatile protein with so many functions has a very complex regulatory system. It includes numerous actin binding proteins and almost a hundred post-translational modifications, such as acetylation and oxidation", says first author Varland. Historically, actin research has centered on understanding the functions of actin binding proteins. The modifications were often ignored.
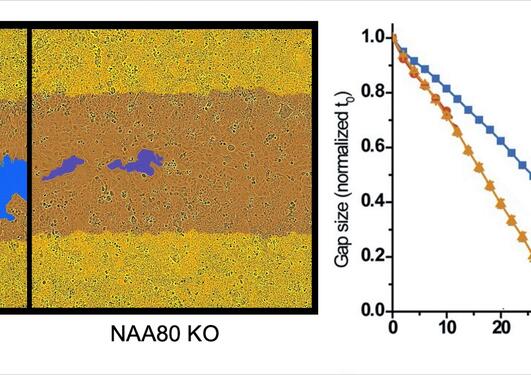
"It is like in the classic fairytale about Cinderella, who serves as maid in her own house and suffers neglect", compares Drazic. Only in the last decade has the importance of actin modifications in regulating the cytoskeleton been recognized. The functions of all these modifications are not clear, as only a few have been studied in detail. These studies demonstrated that modifications affect actin's ability to polymerize into filaments or interact with the abovementioned actin binding proteins. They thus participate in the very delicate fine-tuning of the cytoskeleton.
"These modifications do not occur all at the same time and they are very dependent on the cellular conditions, to come back to the Cinderella story: it is a bit like finding the right shoe that fits at the right moment", elaborates Adrian Drazic.
In this review, the authors argue that these modifications act as integral parts of actin dynamics and should no longer be neglected.
The last comprehensive review on actin modifications was published in 2013 and with new mass spectrometry techniques developed in the last years that are specifically focusing on identifying protein modifications, also numerous new actin post-translational modifications have been identified. "It was about time for a new update on this topic", emphasizes Sylvia Varland.
The authors explicitly want to thank Thomas Arnesen for his support and advice.