Rentzsch Group
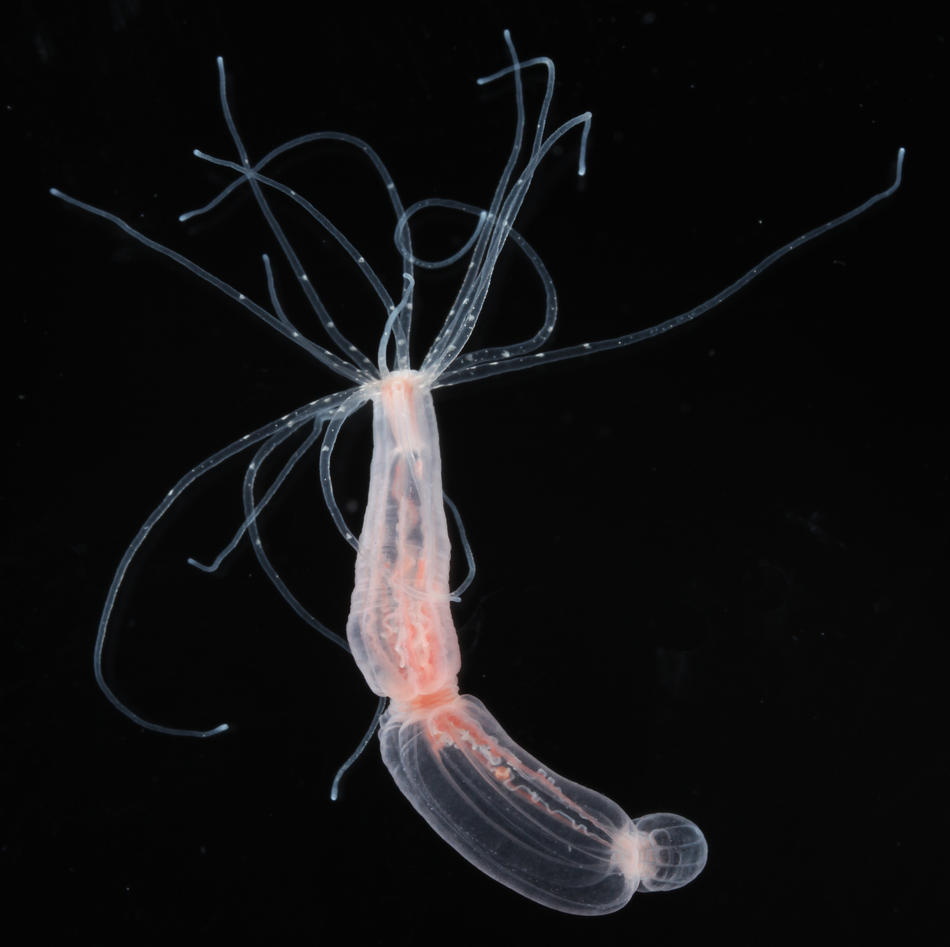
The group is using the sea anemone Nematostella vectensis as a model organism for studying the development and regeneration of the nervous system.

Hovedinnhold
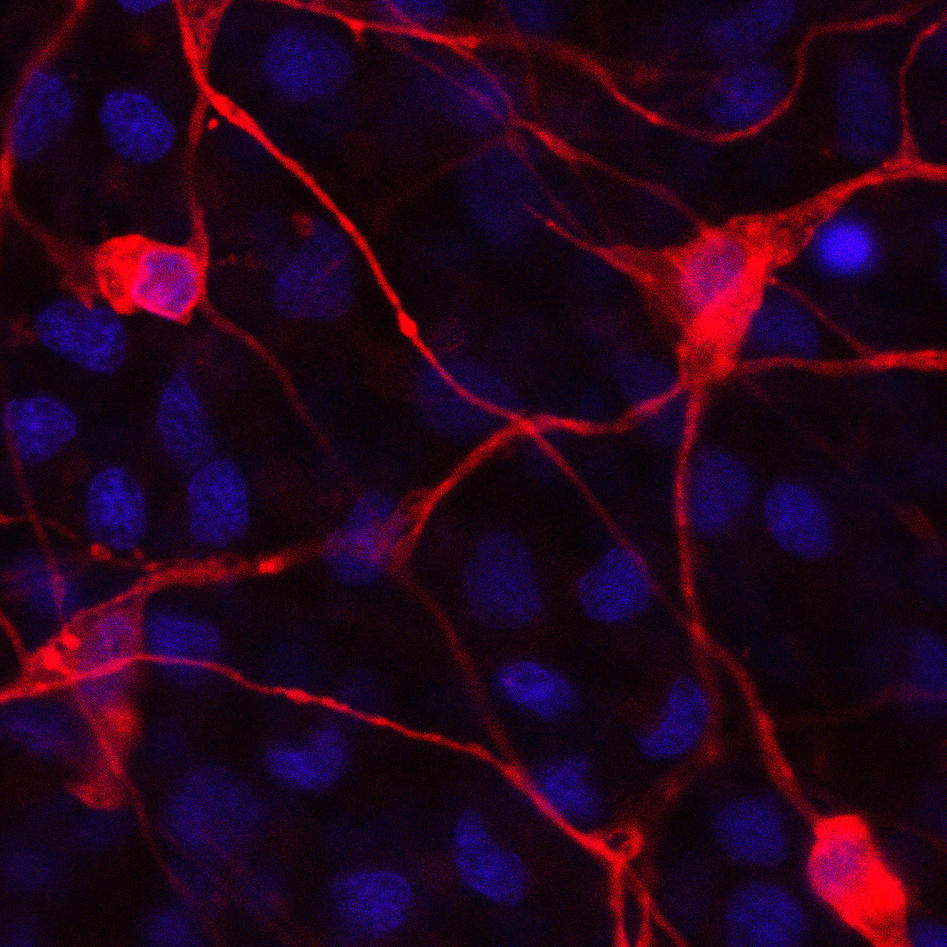
Nervous systems allow animals to sense external stimuli and to monitor their own body functions in order to elicit coordinated behaviour. The central elements that constitute a simple nervous system are sensory structures connected to a specific cell type, the neuron, which can in turn evoke the response of effector cells, e.g. muscle cells. How these central elements arose during evolution and which molecular pathways govern their development in early branching animals is currently poorly understood. We address these questions by studying the cellular and molecular basis of the development of nerve cells and their intercellular connections in the cnidarian Nematostella vectensis.
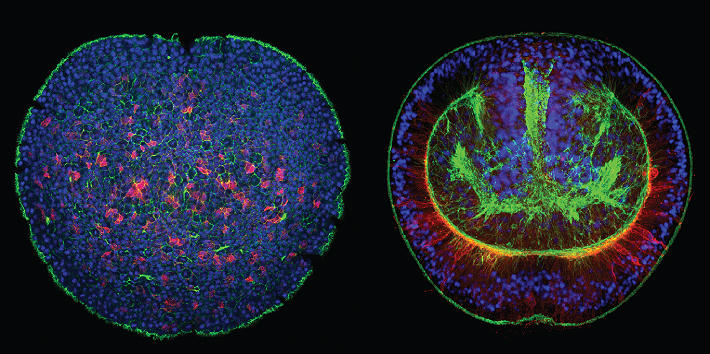
Cnidarians (e.g. corals, sea anemones and jellyfish) are the sister group to the bilaterians (which includes for example vertebrates and arthropods) and one of the earliest branching animal taxa that possesses a nervous system. Understanding neural development in cnidarians and comparing it to bilaterians will help to understand early steps in nervous system evolution. We have recently used transgenic lines and gene knockdown experiments to identify a population of neural progenitor cells that can give rise to all types of neural cells in Nematostella. The work of our and other groups has shown that some key aspects of neurogenesis in Nematostella are controlled by the same genes as in bilaterians. These studies have also shown that Nematostella has a remarkable capacity to generate nerve cells: in contrast to bilaterians, the entire tissue can produce different types of neurons during embryonic development and regeneration.
Our current work aims at understanding the molecular and cellular events that govern the extraordinary neurogenic potential of this seemingly simple animal. What is the developmental potential of individual neural progenitor cells? Which cells give rise to these progenitor cells? What are the transcriptional and epigenetic changes that direct the development of a neural progenitor into a differentiated nerve cell? How is the neurogenic program reactivated during nervous system regeneration? How do neurites find their way to the cells that they innervate?
Since Nematostella is amenable to molecular manipulations (e.g. gene knockdown, transgenics, genome editing) we hope that our work can contribute to a more detailed understanding of the core principles of nervous system development, how neurogenesis may have looked like early in animal evolution and why some animals can regenerate their nervous system better than others.