Novel regulatory mechanism of dopamine synthesis
Our group Biorecognition, along with collaborators in Madrid and Bilbao, Spain, has published a exciting new study by Mary Dayne S. Tai et al. in Nature Communications (link below).

Main content
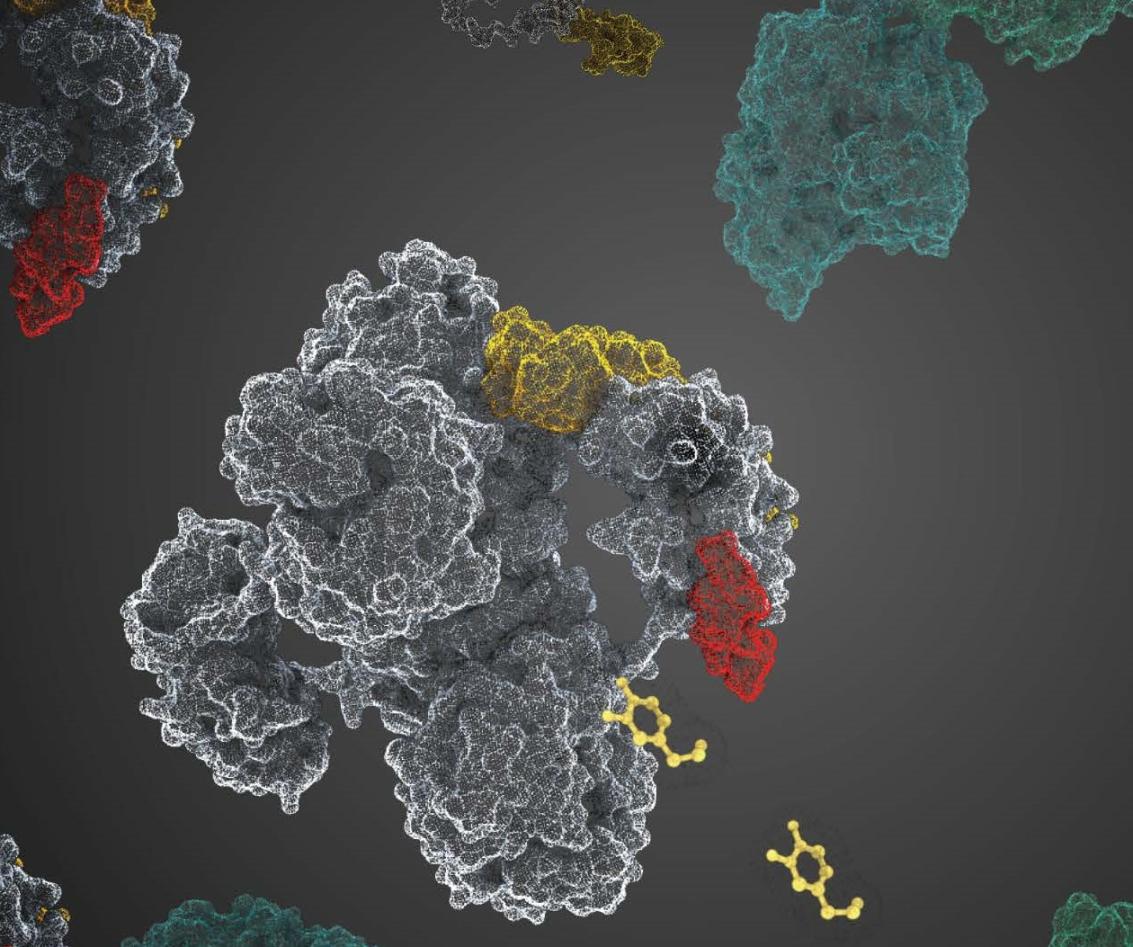
The study presents a new regulatory mechanism behind dopamine biosynthesis, highlighting the role of DNAJC12 in stabilizing tyrosine hydroxylase (TH), the rate-limiting enzyme in the synthesis of dopamine. DNAJC12 prevents TH aggregation, preserves its activity, and stimulates Hsc70 ATPase activity. Cryo-EM imaging and cross-linking mass spectrometry reveal the structural determinants for the formation of the TH:DNAJC12 complex, ensuring that TH active sites remain available for catalysis. Notably, the C-terminal region of DNAJC12 is crucial for the binding to TH, explaining the pathogenic mechanism of the DNAJC12 variant p.Trp175Ter, which is common in DNAJC12 deficiency, an inherited condition that results in low dopamine levels and parkinsonism.